Sars Cov 2 Vaccine Vero Cell Inactivated Side Effects : Sinopharm Bibp Covid 19 Vaccine Wikipedia
The WHO Strategic Advisory Group of Experts SAGE on Immunization has issued Interim recommendations for the use of the inactivated COVID-19 vaccine Sinovac-CoronaVac developed by SinovacChina National Pharmaceutical Group. The primary safety outcome was the combined adverse reactions 7 days after each injection and the primary immunogenicity outcome was neutralizing antibody response 14 days after the whole-course vaccination which was measured by a 50 plaque reduction neutralization test against live severe acute respiratory syndrome coronavirus 2 SARS-CoV-2.

Eu Regulator Begins Real Time Review Of First Chinese Covid 19 Vaccine Reuters
Its easy storage requirements make it highly suitable for low-resource settings.

Sars cov 2 vaccine vero cell inactivated side effects. The most common systematic adverse reaction in the vaccine recipient group was fever seven 2 of 336. COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date. Data collection for final analysis is pending.
151 In June 2021 a report revealed that the UB-612 vaccine developed by the US-based COVAXX was a venture initiated for profits by the Blackwater founder Erik Prince. COVID-19 Vaccine Vero Cell Inactivated COMPOSITION Active ingredient. 2627 The ADE phenomenon has been reported in studies of Middle East respiratory syndromeCoV and SARS-CoV vaccines in animal challenge models.
Wait until these effects have worn off. A possible side effect concern with inactivated virus vaccines is enhanced disease when a vaccine receiver is infected Griffin said. Meanwhile the results of the present study showed that after repeated inoculations with SARS-CoV-2 inactivated vaccine Vero cells.
One concern about COVID-19 vaccines is the antibody-dependent enhancement ADE phenomenon that vaccine could make the subsequent SARS-CoV-2 infection more severe. If you feel unwell do not drive or use machines. 2627 However this was not observed in the preclinical study in an immunization-challenge model of rhesus macaques using the same vaccines.
Main outcomes and measures. And even if a vaccine features the whole SARS-CoV-2 mutations do not just happen in the spike protein but everywhere in the virus Bottazzi added. In this prespecified interim analysis of a randomized clinical trial treatment of adults with either of 2 inactivated SARS-CoV-2 vaccines significantly reduced the risk of symptomatic COVID-19 and serious adverse events were rare.
The emergency use approval of SARS-CoV-2 Vaccine Vero Cell Inactivated CoronavacProduct InformationEnglishInformation on the safety of SARS-CoV-2 Vaccine Vero Cell Inactivated Coronavac is discussed in their comprehensive Risk Management PlanFor more information on reporting side. CoronaVac has no known effect on the ability to drive and use machines. Factsheet for Vaccination of CoronaVac COVID -19 Vaccine Vero Cell Inactivated.
These results indicate that the experimental vaccine might have a low risk of causing an autoimmune response. The most common injection site adverse reaction in the vaccine recipient group was pain 53 16 of 336 and was higher than the placebo group four 4 of 112. What is CoronaVac and what it is used for1.
However some of the side effects see section Side Effectsmay temporarily impact your ability to drive and use machines. Fatigue fever headache and aching limbs are also not uncommon in the first three days after vaccination. Sinovacs Coronavac SARS-CoV-2 Vaccine Vero Cell Inactivated Announces Approval for Phase III Clinical Trial in Adolescents and Children.
This article provides a summary of the interim recommendations. These might include swelling pain redness an itchy rash and other mild forms of. The vaccine is authorized for use under the Prevention and Control of Disease Use of Vaccines Regulation Cap.
CoronaVac is indicated for active immunization against COVID-19 disease caused by SARS-CoV-2 virus. SARS-CoV-2 Vaccine Vero Cell Inactivated CoronavacOn 22 February 2021 the Food and Drug Administration FDA issued authorization granting IP BIOTECH INC. Here is what you need to know.
The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. Previous severe allergic reactions to vaccination such as acute allergic reactions urticaria dyspnea angioneurotic edema or abdominal pain or allergy to known ingredients of inactivated SARS CoV 2 vaccine have occurred. These normal vaccine reactions are usually mild and subside after a few days.
The interim recommendations and the background. Disodium hydrogen phosphate dodecahydra te sodium dihydrogen phosphate monohydrate sodium chloride DESCRIPTION CoronaVac is a milky-white suspension. A person might also experience side effects around the injection site which is usually the upper arm.
Inactivated SARS-CoV-2 Virus CZ02 s train Adjuvant. Named Carnivac-Cov it is an inactivated vaccine for carnivorous animals including pets aimed at preventing mutations that occur during the interspecies transmission of SARS-CoV-2. Have a history of convulsion epilepsy encephalopathy or mental illness or family history.

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Sinopharm Vaccine Side Effects Price Efficacy Of China S Sinopharm Covid Vaccine India News Times Of India
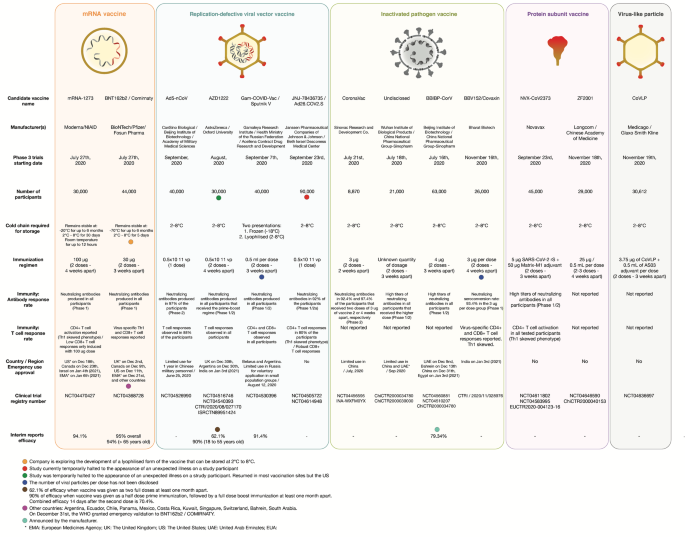
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Coronavirus How Effective Are The Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 01 02 2021
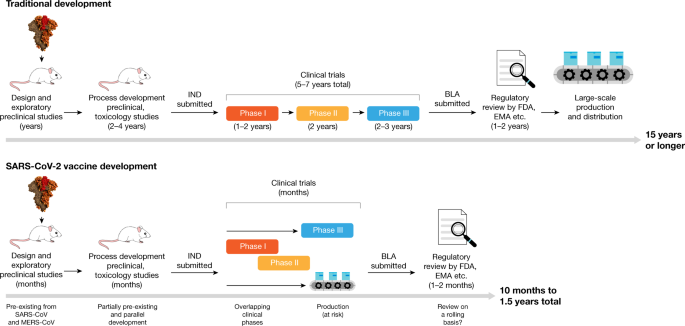
Sars Cov 2 Vaccines In Development Nature
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Covid Bolsonaro Hails Suspension Of Chinese Vaccine Trial Bbc News

Full List Of Adverse Reactions From China S Sinopharm Vaccine Revealed Taiwan News 2021 01 11 09 17 00

Development Of An Inactivated Vaccine Candidate Bbibp Corv With Potent Protection Against Sars Cov 2 Sciencedirect

Integrated Control Of Covid 19 In Resource Poor Countries International Journal Of Infectious Diseases
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
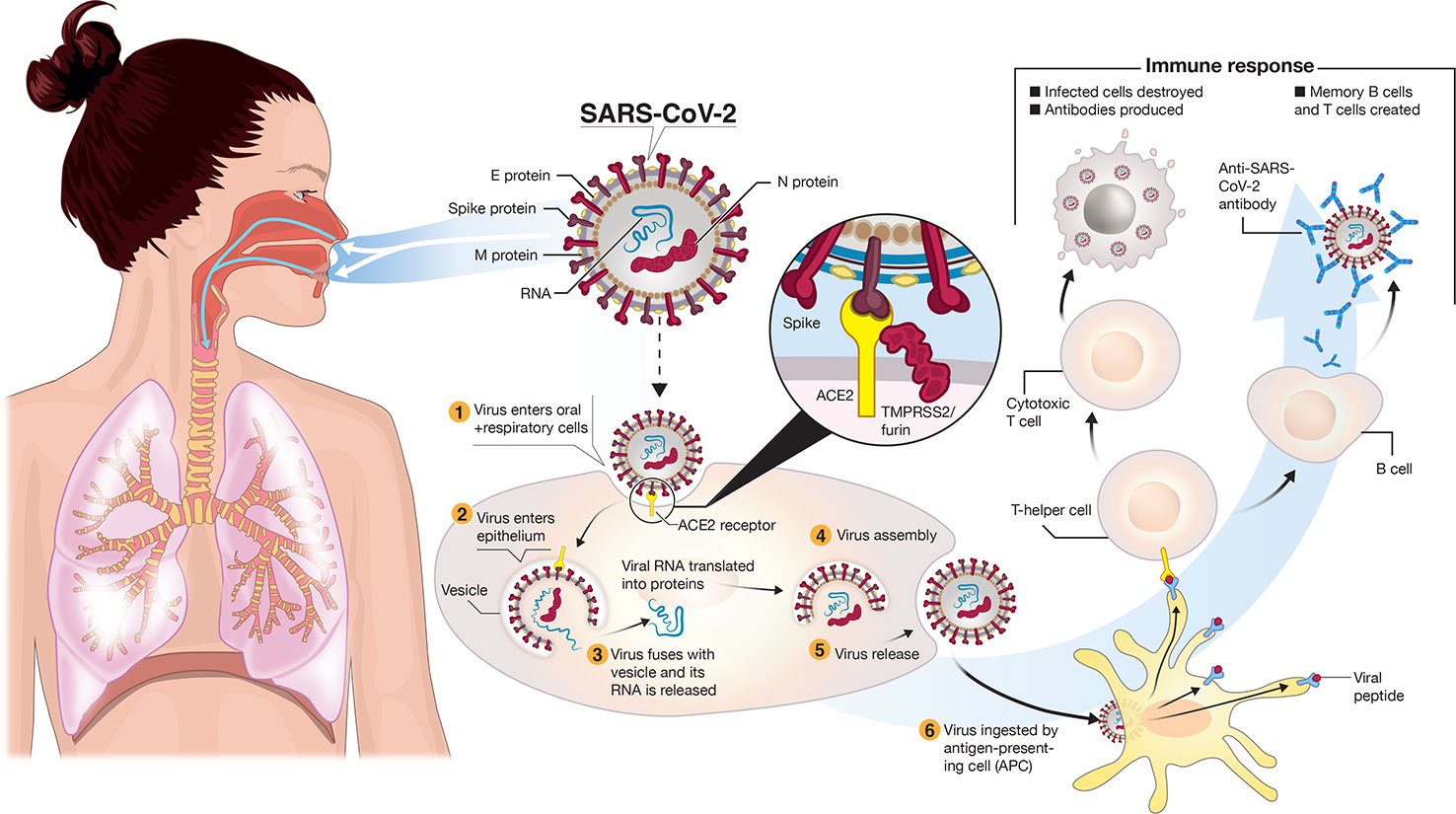
Frontiers A Snapshot Of The Global Race For Vaccines Targeting Sars Cov 2 And The Covid 19 Pandemic Pharmacology

Study Chinese Covid Shot May Offer Elderly Poor Protection
Voa Twisted Words On Sinopharm Vaccine To Support Own Narrative To Discredit Chinese Products Expert Global Times

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Development Of An Inactivated Vaccine Candidate For Sars Cov 2 Science
What Is Vero Cell Ema Launches Rolling Review Of Chinese Covid 19 Vaccine Euronews

